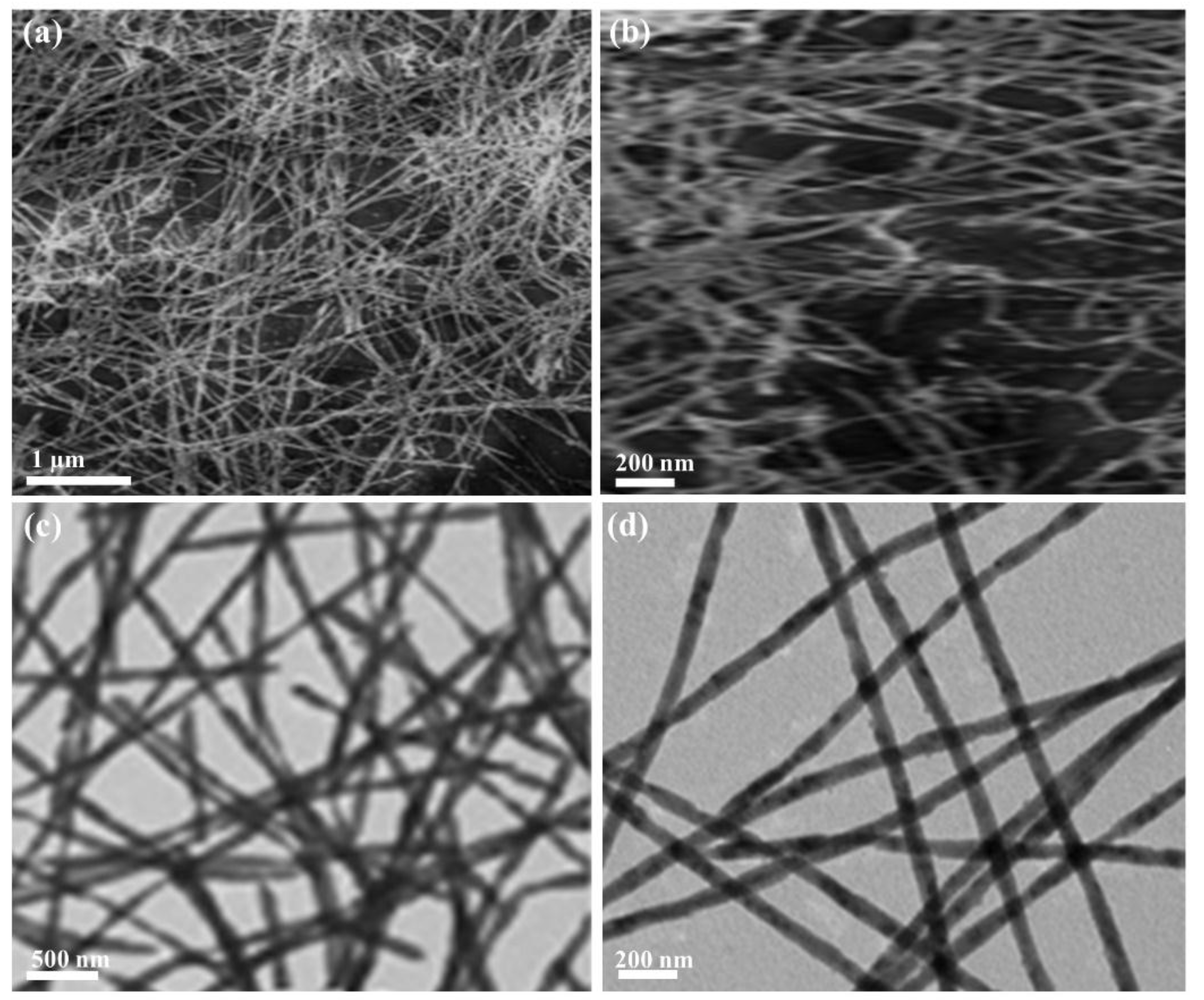
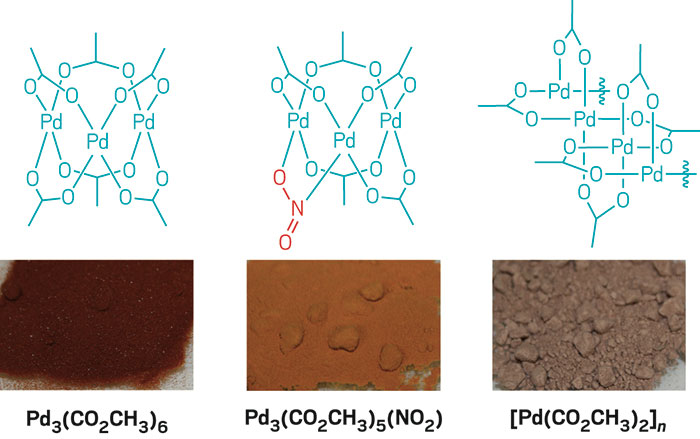
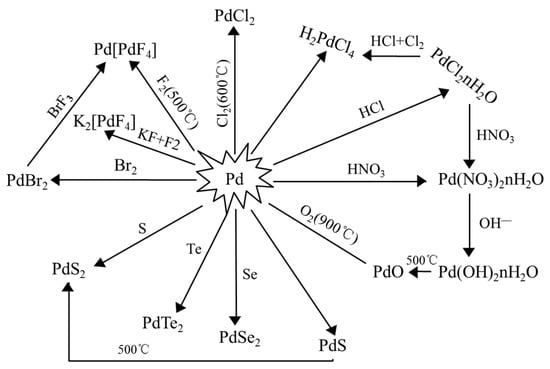
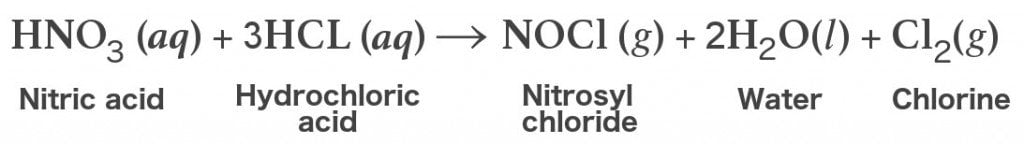
Effect of HNO3 concentration on a novel silica-based adsorbent for separating Pd(II) from simulated high level liquid waste | Scientific Reports

of residue obtained after leaching nitric acid residue with aqua-regia... | Download Scientific Diagram

Palladium(II) nitrate solution 10 wt.% in 10 wt. % nitric acid, 99.999% Palladium(II) nitrate solution 10 wt.% in 10 wt. % nitric acid, 99.999% Manufacturers, Suppliers, Price | India, China
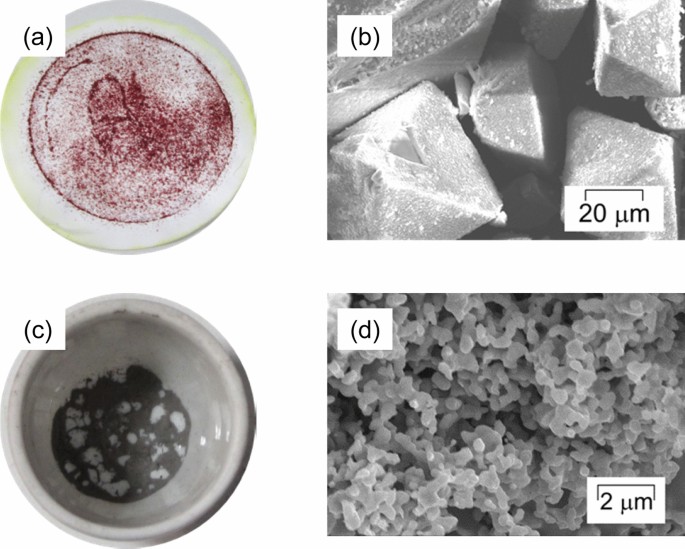
Fundamental Study of Palladium Recycling Using “Dry Aqua Regia” Considering the Recovery from Spent Auto-catalyst | SpringerLink

Recovery of palladium(II) from strong nitric acid solutions relevant to high-level liquid waste of PUREX process by solvent extraction with pyrazole-pyridine-based amide ligands - ScienceDirect

How palladium inhibits CO poisoning during electrocatalytic formic acid oxidation and carbon dioxide reduction | Nature Communications

Spectroscopic and first-principles calculation studies of the chemical forms of palladium ion in nitric acid solution for development of disposal of high-level radioactive nuclear wastes: AIP Advances: Vol 8, No 4
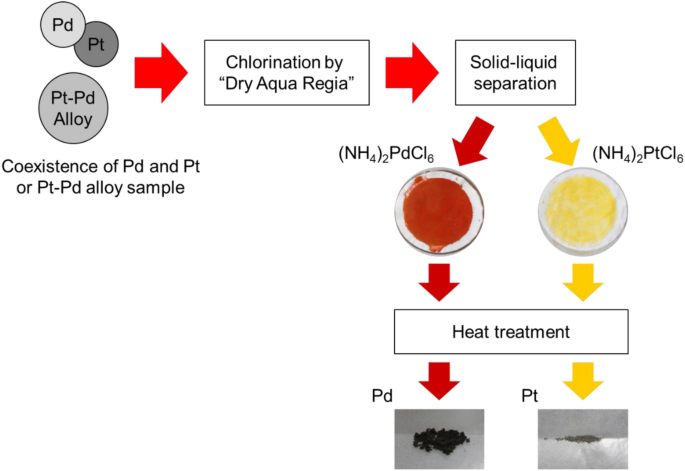
Fundamental Study of Palladium Recycling Using “Dry Aqua Regia” Considering the Recovery from Spent Auto-catalyst | SpringerLink
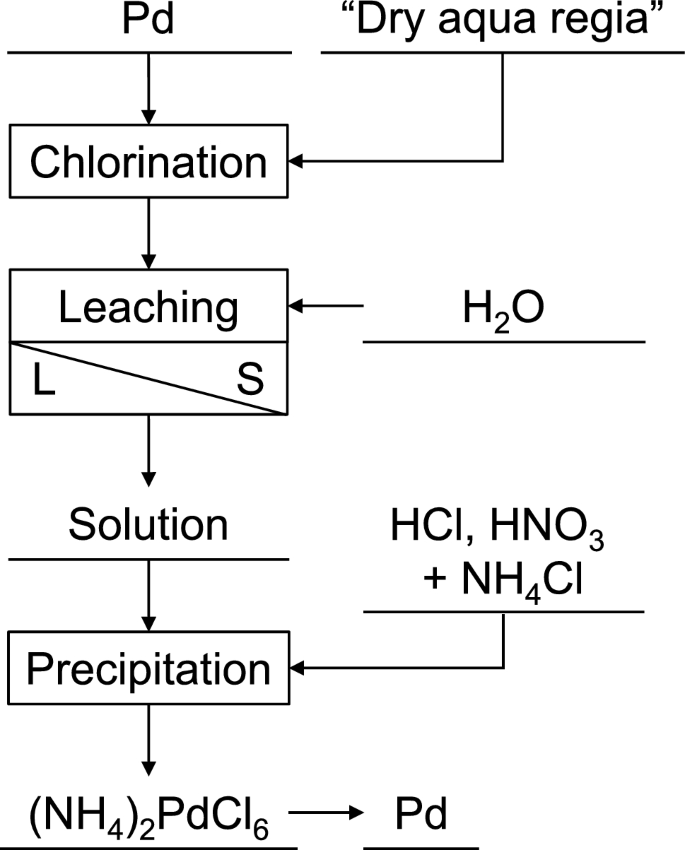
Fundamental Study of Palladium Recycling Using “Dry Aqua Regia” Considering the Recovery from Spent Auto-catalyst | SpringerLink

UV-vis absorption patterns of palladium (II) in various concentrations... | Download Scientific Diagram

Palladium(II) nitrate solution 10 wt.% in 10 wt. % nitric acid, 99.999% Palladium(II) nitrate solution 10 wt.% in 10 wt. % nitric acid, 99.999% Manufacturers, Suppliers, Price | India, China
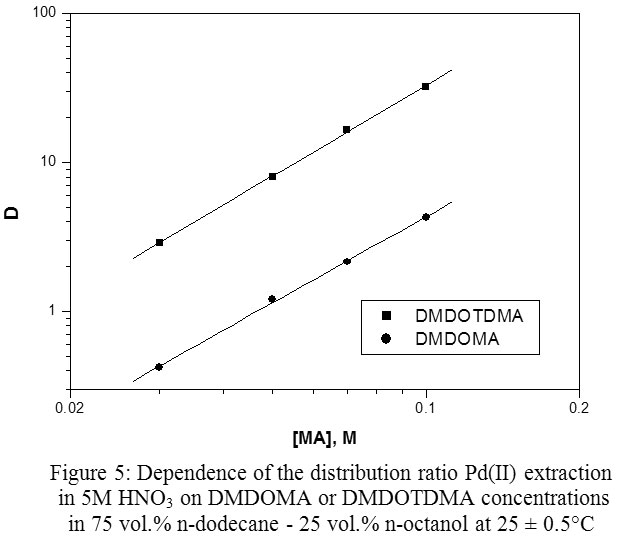
Recovery of Palladium from Concentrated Nitrate Solutions with N,N′‐Dimethyl‐N,N′‐Dioctyltetradecylmalonamide as new Extractant : Oriental Journal of Chemistry

Palladium(II) nitrate solution 10 wt. % in 10 wt. % nitric acid, 99.999% trace metals basis | Sigma-Aldrich
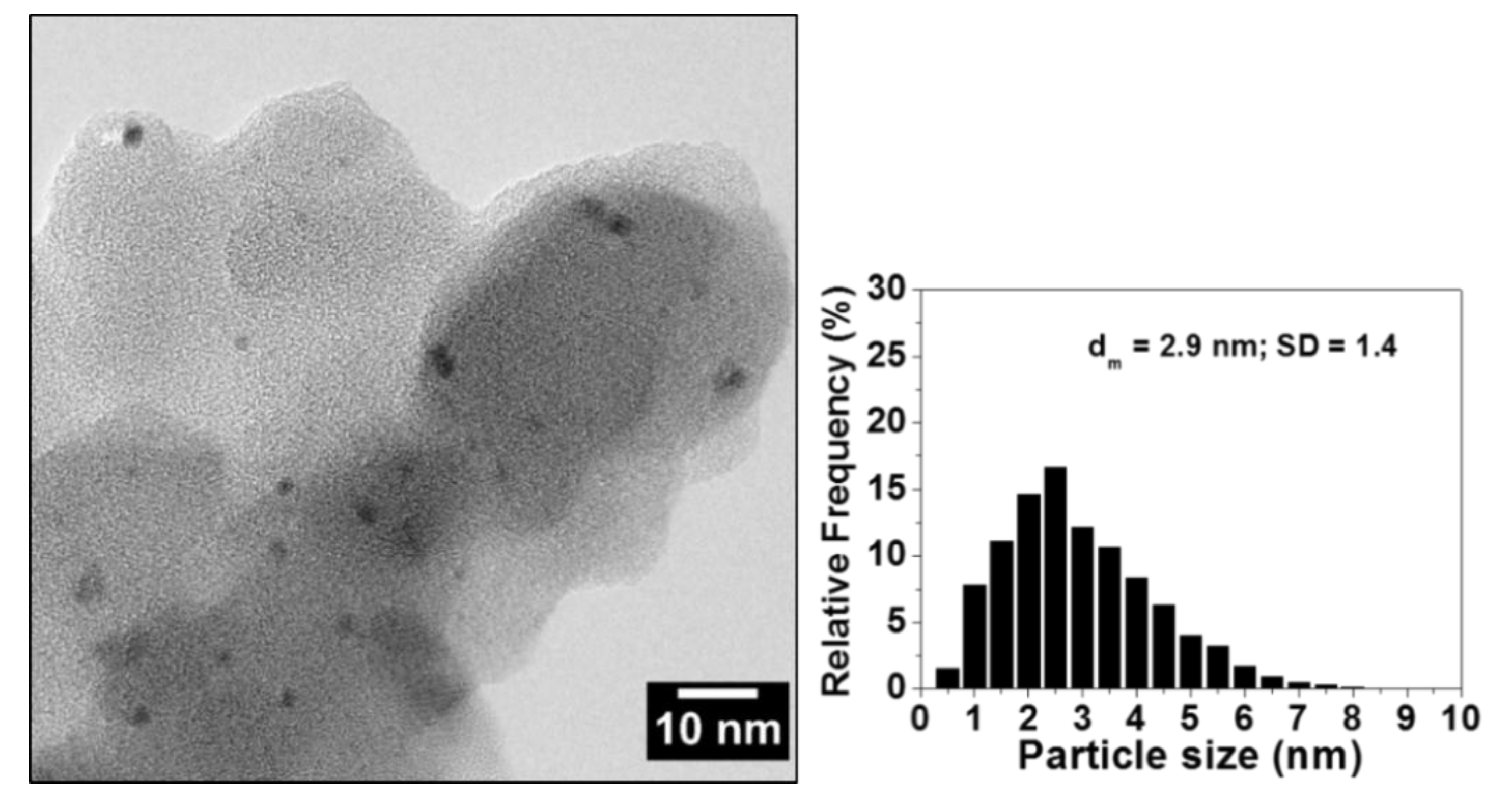
Catalysts | Free Full-Text | Polyvinylpyridine-Supported Palladium Nanoparticles: An Efficient Catalyst for Suzuki–Miyaura Coupling Reactions
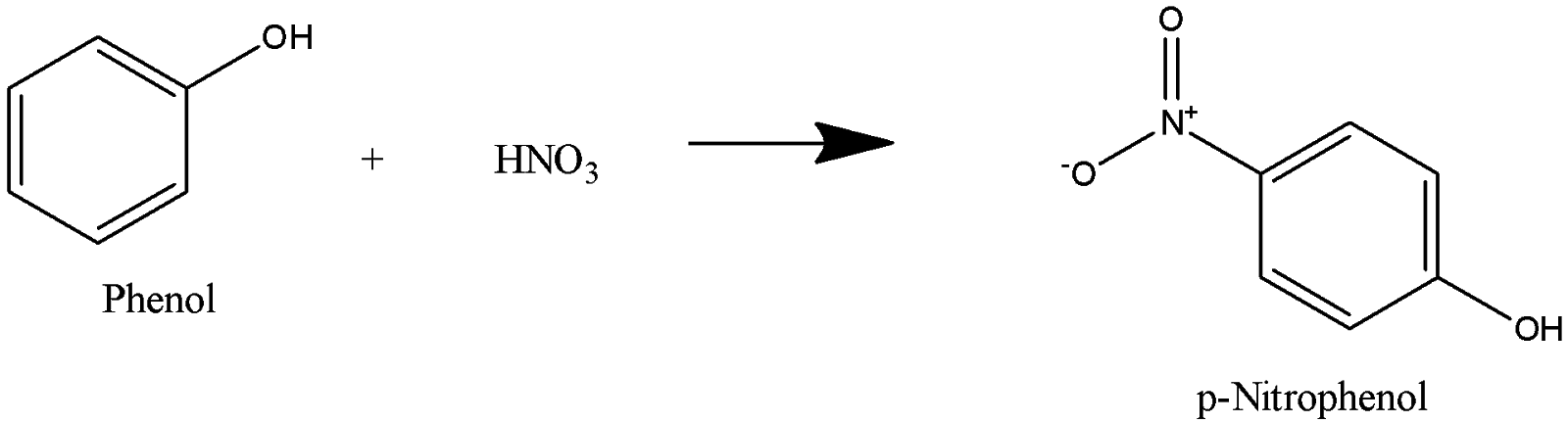
Which of the following sets of reactants is used for the preparation of paracetamol from phenol?(A) $HN{{O}_{3}},{{H}_{2}}\/Pd,{{(C{{H}_{3}}CO)}_{2}}O$(B) ${{H}_{2}}S{{O}_{4}},{{H}_{2}},Pd,{{(C{{H}_{3}}CO)}_{2}}O$(C) ${{C}_{6}}{{H}_{5}}{{N}_{2}}Cl,SnC ...